

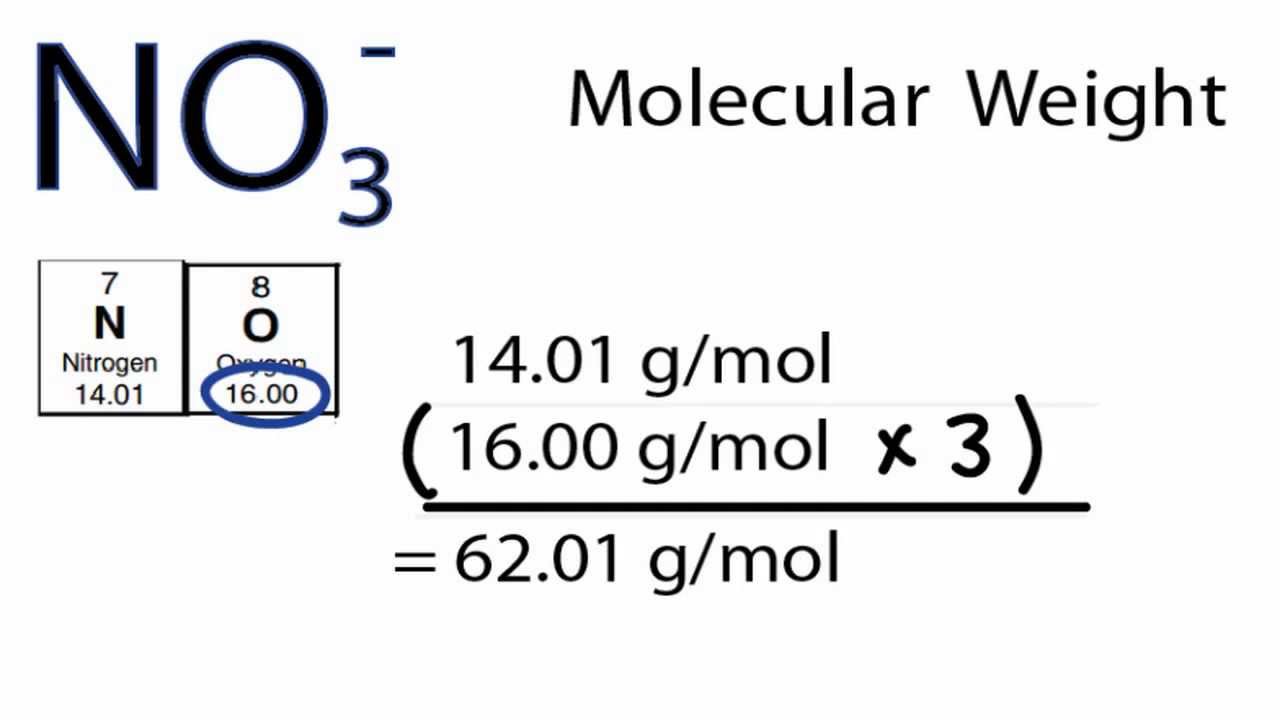
The balanced chemical equation is written by using the given reaction statement and calculated the mass NaOH of required for the 0.147g of oxalic acid, which is used as a primary standard in the standardization of NaOH. Let required weight of NaOH for standardizing be ‘X’. The balanced chemical equation is given below:Īccording to the balanced chemical equation, it is clear that 126.07g of oxalic acid required for standardizing the 80 grams of NaOHĠ.147g of oxalic acid is used as a primary standard for standardizing the NaOH 2L of 0.2M H2SO4 is reacted with 2L of 0.1M NaOH solution, the molarity of. Oxalic acid is often used as a primary standard for the standardization for NaOH. The density of a monobasic strong acid (Molar mass 24.2 g/mol) is 1.21 kg/L.
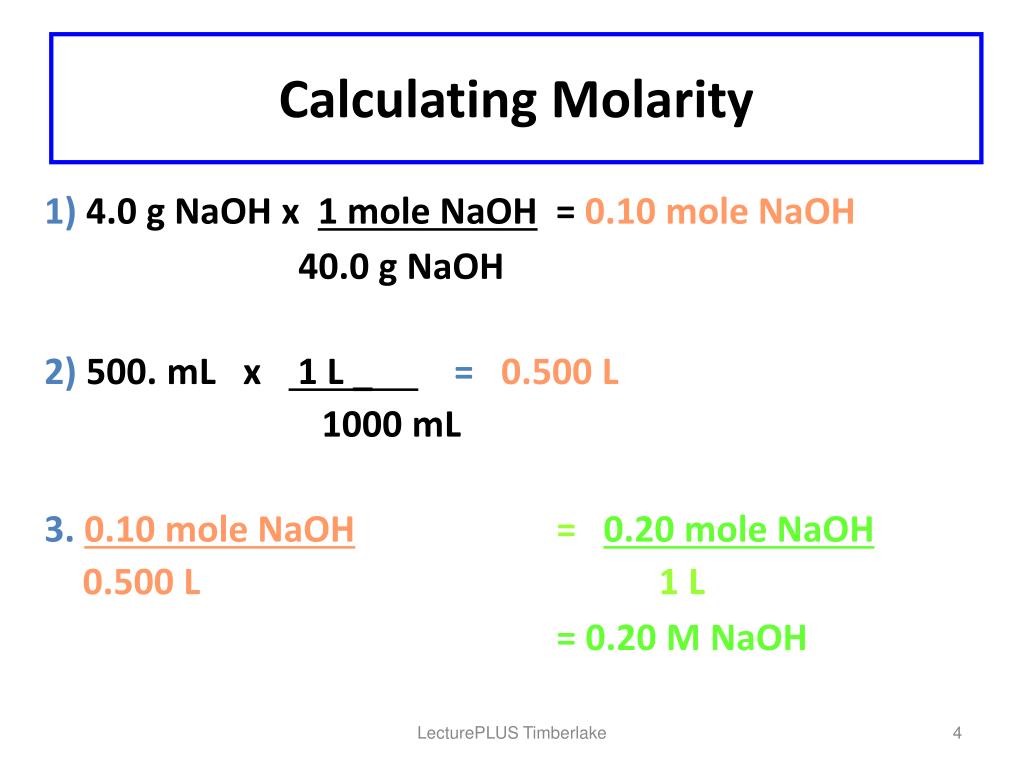
Molarity: The number of moles of solute present in unit volume of solution. **Molar mass:**The mass of one mole of compound is called molar mass. Law of conservation of mass: mass is neither created nor destroyed in a chemical reaction which states that the mass of the reactants before the reaction is equal to the mass of the products after the reaction.īalanced chemical equation: this is an application for the law of conservation of mass which states that the number of atoms presents on the reactant side is equal to the atoms present in the product side. Thebalanced chemical equation can be obtained using this phenomenon.Mass and molar concentration can be calculated by using this balanced chemical equation. Law of conservation of mass states that, in a chemical reaction, mass is neither created nor destroyed. If 0.147 g of oxalic acid dihydrate is nutralizedby 23.64mL of a NaOH solution, what is the molar concentration ofthe NAOH solution? Oxalic acid is a diprotic acid. Oxalic acid, H2C2O4*2H2O (molar mass = 126.07 g/mol) is oftenused as a primary standard for the standardization of a NaOHsolution.


 0 kommentar(er)
0 kommentar(er)
